Problem 12
Formula: C7H9N
Spectroscopy Reference

Show Unsaturation answer
C7H9N
Rule 3, omit the N and one H, gives C7H8
7 - 8/2 + 1 = 4 degrees of unsaturation.
Look for an aromatic ring, plus another pi bond or aliphatic ring.
Show IR answer
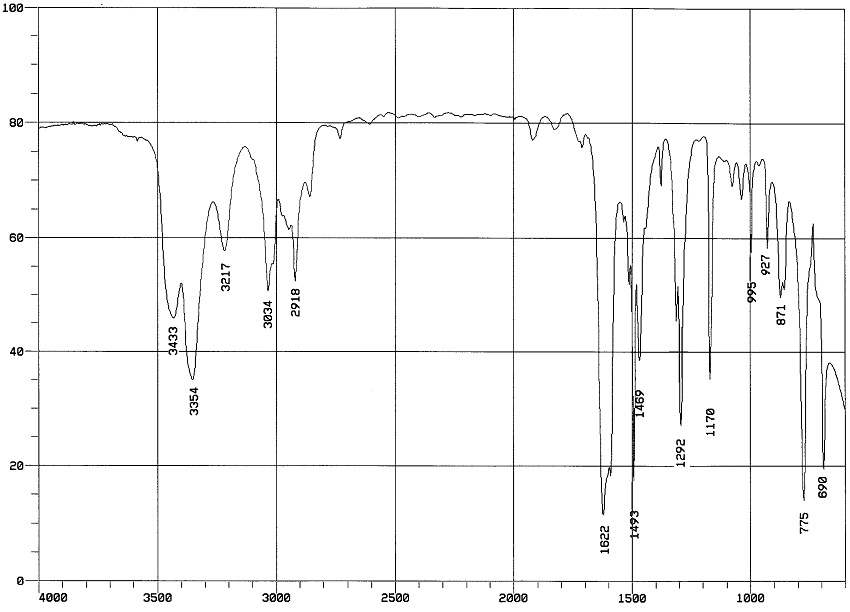
The two bands at 3433 and 3354 indicate a primary amine (-NH2). The bands at 3000-2850 indicate C-H alkane stretches. The band at 3034 indicates aromatic C-H stretch; aromatics also show bands in the regions 1600-1585 and 1500-1400 (C-C in-ring stretch), and 900-675 (C-H out-of-plane). C-N stretch of aromatic amines would show up at 1335-1250 (there is a band in that region).
Show Structure answer

This is the structure. See if you can assign the peaks on your own.
Show NMR answer
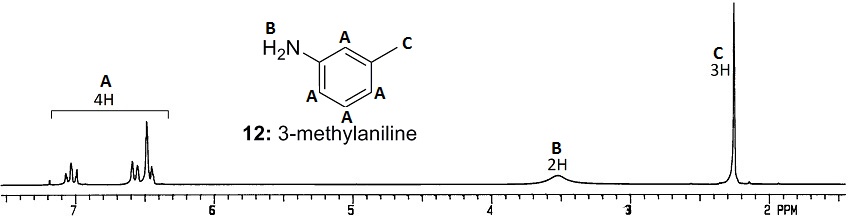
You are not expected to tell the aromatic Hs apart from each other, but you are expected to know that the ring is not para-substituted.
Previous Problem
Problems list
Next Problem


 Organic Chemistry at CU Boulder
Organic Chemistry at CU Boulder