Problem 13
Formula: C5H13N
Spectroscopy Reference


Show Unsaturation answer
C5H13N
Rule 3, omit the N and one H, gives C5H12
5 - 12/2 + 1 = 0 degrees of unsaturation.
No pi bonds or rings.
Show IR answer
The two bands at 3388 and 3292 indicate a primary amine (-NH2). The bands at 3000-2850 indicate C-H alkane stretches.
Show Structure answer

This is the structure. See if you can assign the peaks on your own.
Show NMR answer
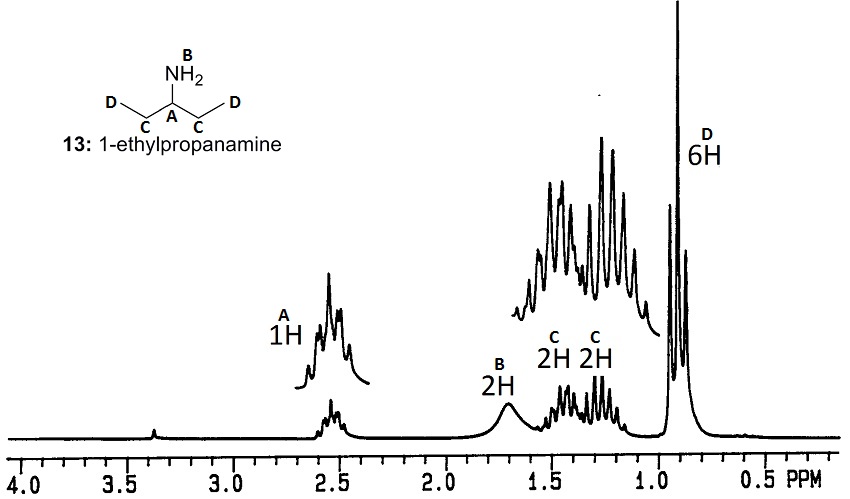
Note that C is showing as 2 2H peaks, instead of a 4H peak. This may be caused by a similar effect as in Problem 4 - on each carbon C, the H closer to the N will be in a slightly different environment. If the rotation of this carbon is hindered, these Hs may have a different chemical shift.
Previous Problem
Problems list
Next Problem


 Organic Chemistry at CU Boulder
Organic Chemistry at CU Boulder