C8H11N
MW 121
Calculate the degree of unsaturation: the answer is 4. The molecule probably has an aromatic ring.
The molecule has a nitrogen, therefore you should look for the N-H stretch of an amine, between 3400-3250. There are two bands in this region, one at 3460 and one at 3373; this indicates that the amine is primary. This is supported by the band at 1622, which is likely the N-H bend of a primary amine. Note the bands just to the left of 3000, indicating aromatic C-H stretch. This supports the degree of unsaturation suggestion that is an aromatic compound.

The NMR spectrum is below:

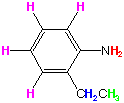
Note the 4 protons at 6.7-7.2 ppm: this indicates there are 4 aromatic protons in the molecule, therefore a di-substituted aromatic ring. The pattern does not look symmetrical, so it is probably ortho or meta (we are giving you the answer: it is ortho). From the IR, we suspect a primary amine; the NMR peak at 3.6 ppm indicates not only an amine, but an aromatic amine. Thus we know this much of the structure so far:

The remaing two peaks should look familiar to you by now: a triplet of 3 protons next to a quartet of 2 protons. This is an ethyl group, -CH2CH3. The structure and correlating NMR spectrum is shown below: