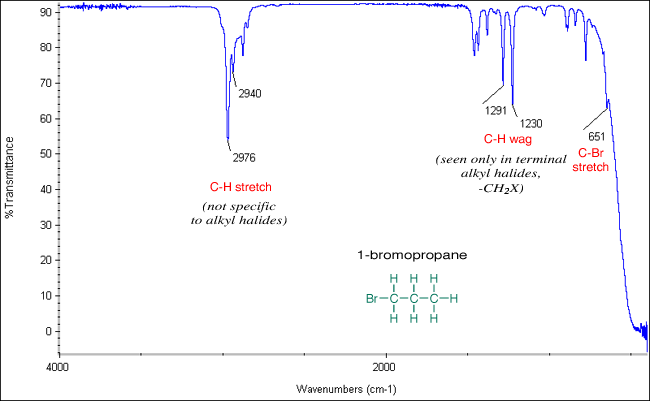

Alkyl halides are compounds that have a C–X bond, where X is a halogen: bromine, chlorine, fluorene, or iodine (usually Br or Cl in the organic chemistry teaching labs). In general, C–X vibration frequencies appear in the region 850-515 cm-1, sometimes out of the range of typical IR instrumentation. C–Cl stretches appear from 850–550 cm-1, while C–Br stretches appear at slightly lower wavenumbers from 690-515 cm-1. In terminal alkyl halides, the C–H wag of the –CH2X group is seen from 1300-1150 cm-1. Complicating the spectra is a profusion of absorptions throughout the region 1250-770 cm-1, especially in the smaller alkyl halides. Note that all of these bands are in the fingerprint region.
In summary, the following bands are specific to alkyl halides:
The spectra of 1-bromopropane and 1-chloro-2-methylpropane are shown below. Note the C–Br or C–Cl stretches in the region 850-515 cm-1. They also show C–Br or C–Cl wag in the region 1300-1150 cm-1.


Even though both 1-bromopropane and 1-chloro-1-methylpropane have similar spectra and the bands that distinguish one from the other are in the fingerprint region, if the two spectra are overlayed, examination of the fingerprint region readily shows that they are different compounds. (Shown below.)