Problem 7
Formula: C8H14O3
Spectroscopy Reference


Show Unsaturation answer
C8H14O3
Rule 2, omit O, gives C8H14
8 - 14/2 + 1 = 2 degrees of unsaturation.
Look for 2 pi bonds or aliphatic rings, or 1 of each.
Show IR answer
The bands at 1745 and 1716 indicate that there are two carbonyls, probably an aliphatic ester and an aliphatic ketone. The bands at 3000-2850 indicate C-H alkane stretches.
Show Structure answer
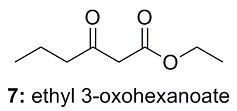
This is the structure. See if you can assign the peaks on your own.
Show NMR answer
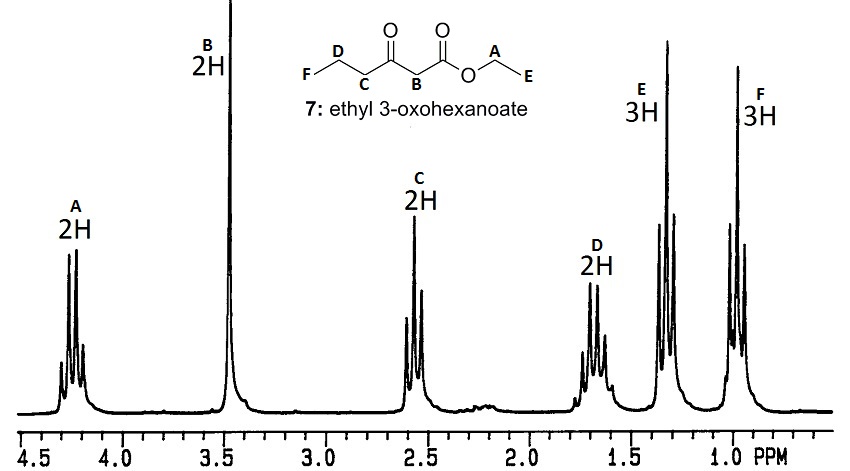
Even though A, B, C and D are all 2H peaks, they can be distinguished by chemical shift and splitting. B is outside the normal range for protons next to carbonyls, because it's adjacent to both carbonyls and the combined deshielding is higher than normal.
Previous Problem
Problems list
Next Problem


 Organic Chemistry at CU Boulder
Organic Chemistry at CU Boulder