Problem 16
Formula: C10H14O
Spectroscopy Reference


Show Unsaturation answer
C10H14O
Rule 2, omit O, gives C10H14
10 - 14/2 + 1 = 4 degrees of unsaturation.
Look for an aromatic ring.
Show IR answer
The broad dip at 3250 indicates an OH group. The peak at 2963 indicates an alipahtic group. The peaks at 1599 and 1514 indicate an aromatic ring.
Show Structure answer
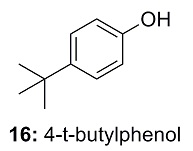
This is the structure. See if you can assign the peaks on your own.
Show NMR answer

You are not expected to tell the aromatic Hs apart from each other, but you should be able to tell that the ring is para-substituted.
Previous Problem
Problems list
Next Problem


 Organic Chemistry at CU Boulder
Organic Chemistry at CU Boulder