

Cleavage of M+ (also written M+.) ions from electron impact ionization or [M+1]+ ions from chemical ionization at bonds near the site of ionization gives smaller fragment ions. The appearance of fragment ions in the mass spectrum and an understanding of fragmentation mechanisms can provide information about the structure of unknown compounds. Electron impact ionization yields molecular radical ions, also called odd electron ions. Their fragmentation is very common and is often predicted through an understanding of free radical chemistry. Chemical ionization yields even electron ions that are less prone to fragmentation. The fragmentation that does occur is often predicted through an understanding of cation chemistry. A general rule is that the most favored fragmentation pathway cleaves the weakest bond or bonds near the site of ionization to give the most stable cation and/or radical products. Mechanisms for odd electron fragmentation are written with fish hook arrows to show the migration of single electrons, and mechanisms for fragmentation of even electron ions, with double headed arrows to show the migration of electron pairs.
1. If one bond of an odd electron ion cleaves, the products are an even electron cation and an odd electron neutral radical. Only the cation is detected.

2. If two bonds of an odd electron ion cleave, either simultaneously of sequentially, the products are an odd electron cation and an even electron neutral fragment.

3. If one (or even two bonds) of an even electron ion cleaves, the products are an even electron cation and an even electron neutral fragment.

4. Odd electron ions can also undergo structural (also called skeletal) rearrangement of their atoms prior to fragmentation. Rearrangements are sometimes predictable but often make mass spectral fragment ion interpretation difficult.

5. Fragment ions can undergo additional fragmentation to give smaller fragment ions. In terms of structure determination, the most important fragment ions are those of higher m/z that result from fragmentation of the molecular ion. Identification of the parent ion, the ion undergoing fragmentation, as the molecular ion or a fragment ion undergoing subsequent fragmentation is not always simple. More advanced mass spectral techniques facilitate this identification.
Figure 8. Examples of one bond fragmentation mechanisms for odd electron ions to give even electron ions. Radical cations are created by electron impact ionization as shown in Figure 5. In general, fragmentation occurs one bond removed from the radical site of the radical cation. Each fish hook arrow shows the migration of a single electron. When two fish hook arrows come together, a new bond is formed; when two fish hook arrows move apart, a bond is broken. Dashed lines show which bonds are broken.

Figure 9. Examples of two bond fragmentation mechanisms for odd electron ions to give odd electron ions. Radical cations are created by electron impact ionization as shown in Figure 20.4. Each fish hook arrow shows the migration of a single electron. When two fish hook arrows come together, a new bond is formed; when two fish hook arrows move apart, a bond is broken. Dashed lines show which bonds are broken.

Figure 10. Examples of one bond fragmentation mechanisms for even electron ions created by chemical ionization with CH5+ as shown in Figure 6. Each double headed curved arrow shows the migration of an electron pair. Dashed line shows which bond is broken. Fragmentation usually occurs through the loss of a small stable molecule such as water.

Application of fragmentation mechanisms for the assignment of some of the higher m/z fragment peaks in appearing in EI mass spectra are shown in Figures 11, 12, and 13 for a ketone, and amine and an ester, respectively. Try writing the mechanisms with fish hook arrows using Figures 8 and 9 as a guide.
Figure 11. EI/MS of 2-pentanone with assignment of some one bond and two bond cleavages using mechanisms in Figures 8 and 9.
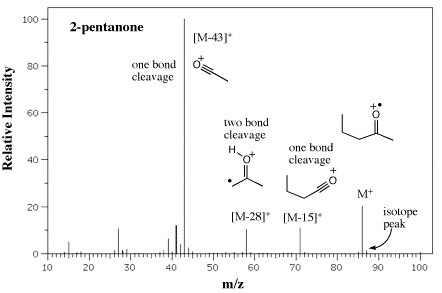
Figure 12. EI/MS of N-ethylbutylamine with assignment of some one bond cleavages using one of the mechanisms in Figure 8.

Figure 13. EI/MS of ethyl butanoate (ethyl butyrate) with assignment of some one and two bond cleavages using the mechanisms in Figures 8 and 9.

Next section: Instrumentation: Notes and Acknowledgements
![]()
Copyright information: Original content © University of Colorado, Boulder, Chemistry and Biochemistry Department, 2011. The information on these pages is available for academic use without restriction.